New scientific article published
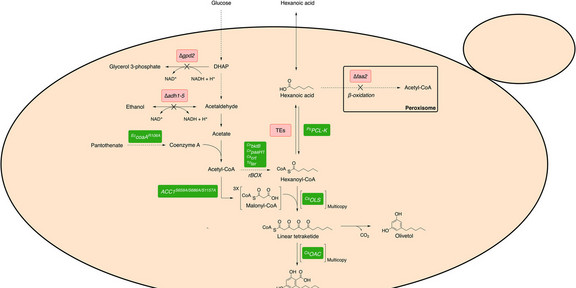
Cannabinoids comprise a large class of bioactive compounds found primarily in the plant species Cannabis sativa and are of interest due to their pharmacological and therapeutic potential. The aromatic polyketide, olivetolic acid (OA), is a major precursor in the cannabinoid biosynthesis pathway and is derived from hexanoyl-CoA and malonyl-CoA by the action of olivetol synthase (OLS) and olivetolic acid cyclase (OAC). To date, most microbial cannabinoid production systems rely on the external supplementation of hexanoic acid together with the overexpression of acyl-activating enzyme 1 from C. sativa(CsAAE1) to provide hexanoyl-CoA. Here, we implement a heterologous OA biosynthesis pathway into the yeast Saccharomyces cerevisiae and describe various biochemical and metabolic engineering strategies to overcome the need for external hexanoic acid supplementation. We ensured a sufficient endogenous supply of hexanoyl-CoA and further optimized OA production by enhancing precursor supply. Furthermore, we present a mutant phenylacetate-CoA ligase derived from Penicillium chrysogenum(PcPCL-K) which displayed superior hexanoyl-CoA ligase activity to CsAAE1. Together, these strategies enabled the production of 180 mg/L OA using shake flask cultures. Our results provide details of key metabolic engineering steps required for the biosynthetic production of cannabinoids and their precursors in S. cerevisiae.